Original Research
The Impact of Institutional Clinical Care Guidelines on Treatment Outcomes in Pediatric Musculoskeletal Infection: A Systematic Review
1Department of Orthopaedic Surgery, BC Children’s Hospital, Vancouver, BC, Canada; 2Department of Orthopaedics, The University of British Columbia, Vancouver, BC, Canada
Correspondence: Andrea Simmonds, MD, MHSc, FRCSC, BC Children’s Hospital, 4480 Oak St., Vancouver, BC, V6H 3V4, Canada. E-mail: [email protected]
Received: June 12, 2023; Accepted: August 30, 2023; Published: November 15, 2023
Volume 5, Number 4, November 2023
Abstract
Background: Pediatric musculoskeletal infections (MSKIs) are complicated to manage, and inconsistent approaches to care within an institution can negatively affect patient recovery. The aim of this systematic review is to assess the impact of implementing institutional clinical care guidelines (CCGs) on treatment outcomes in pediatric MSKIs.
Methods: The authors carried out a systematic review of medical literature using the databases Embase and Medline. Ten comparative studies assessing quantitative treatment outcomes of pediatric patients with MSKIs before and after implementation of a CCG were included. Studies in adult populations and those lacking comparative analysis were excluded.
Results: Implementing CCGs led to improvements in patient care and clinical outcomes. Outcomes assessed across papers varied. Implementation of CCGs for the management of pediatric patients with MSKIs was shown to shorten patients’ length of stay, duration of IV and/or oral antibiotic therapy, and duration of clinical symptoms associated with MSKIs. There was also evidence of reduced financial costs, which was determined by cost-effective analysis in one study. Additionally, improved access to magnetic resonance imaging and better coordination between disciplines was discussed in some studies to benefit patients’ outcomes by providing an earlier diagnosis and the ability to image concerns throughout treatment.
Conclusions: CCGs for pediatric patients with MSKIs improve outcomes by decreasing length of stay and inpatient costs, promoting earlier transition from IV to oral antibiotics, decreasing central line use, encouraging coordination between disciplines, and prioritizing earlier access to MRI and surgery. Further research across existing literature regarding the impact of early access to MRI is of interest for the future.
Level of Evidence: II
Key Concepts
- Use of clinical care pathways to manage pediatric musculoskeletal infections improves patient outcomes.
- Limiting unnecessary use of intravenous antibiotics in pediatric musculoskeletal infections decreases length of stay in hospital and central venous catheter use.
- Clinical care pathways to manage pediatric musculoskeletal infections help decrease length of stay in hospital and overall hospital costs.
- Implementing clinical care pathways to manage pediatric musculoskeletal infections can encourage earlier access to MRI.
Introduction
Musculoskeletal infections (MSKIs) in pediatric populations, such as osteomyelitis, septic arthritis, and pyomyositis, are challenging to treat due to evolving epidemiology and pathological complexity. Disagreement on the most effective method to diagnose and treat MSKIs within an institution can lead to variation in care practice, which negatively impacts patient outcomes.1,2 A clinical care guideline (CCG) (also referred to as a clinical care protocol, clinical practice guideline, care process model, or clinical algorithm) is a standardization of treatment within an institution, which can reduce practice variability and improve patient outcomes.3
While several institutions have utilized CCGs for the treatment of pediatric MSKIs, there is lacking evidence comparing treatment outcomes before and after CCG implementation across multiple institutions.4,5 There is little suggestion as to which treatment outcomes are most commonly or most significantly impacted by the utilization of CCGs across different studies and institutions.
Through this systematic review, we hope to identify treatment outcomes that are significantly impacted—either positively or adversely—by the implementation of a CCG for management of MSKIs in a pediatric patient population. This review will also shine a light on components of CCGs that are of interest, opening up avenues for future research on this topic. We hypothesize that CCGs will allow for faster identification of patients with MSKI, leading to improvement in treatment outcomes such as length of stay and readmission rates.
Materials and Methods
Search Strategy
A search of medical literature regarding CCGs on MSKIs was carried out with the consultation of a librarian at BC Children’s Hospital Research Institute. First, a limited literature search was conducted to identify keywords used in papers of interest to construct a search strategy. A search filter developed at the University of Alberta was incorporated into the search to assist in limiting the results to studies on pediatric populations.6 This search strategy was then applied to two databases, Ovid Medline and Ovid Embase, on April 25, 2022, with subject headings consisting of keywords, relevant variants, and MeSH (Medical Subject Headings). The terms included: osteomyelitis, transient synovitis, clinical care guideline, care pathway, etc. (see supplemental information for full search). The search results were imported to Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia, www.covidence.org), duplicates were removed, and titles and abstracts were assessed independently against the eligibility criteria by two reviewers (S.P. and J.H.). A third reviewer (A.S.) was consulted if consensus regarding eligibility could not be reached. Full texts of the selected articles were then reviewed independently by the initial two reviewers. Studies that met eligibility criteria were included and then subject to data extraction. The Cochrane Tool to Assess Risk of Bias in Cohort Studies was used to assess the risk of bias of the included papers (Tool to Assess Risk of Bias in Cohort Studies. Cochrane Methods Bias, Centre for Evidence-Based Medicine Odense, Odense, Denmark. www.methods.cochrane.org). This protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) on May 10, 2022, with registration number CRD42022323261.
Primary Outcomes Examined Across Studies
Primary outcomes varied across the studies. The focuses (examined before and after guideline implementation) included, but were not limited to, length of stay in the hospital, cost of care, duration and type of antibiotic treatment, timing of switch to oral antibiotics, and identification of the source pathogen. Each study defined statistical significance at p=0.05.
Results
Literature Search
The search strategy yielded 1064 studies after duplicates were removed (Figure 1). Studies were included if they examined a pediatric population from 0 to 17 years of age, compared treatment outcomes of patient populations before and after the implementation of a CCG for management of MSKIs, and contained quantitative analysis of the effects of the intervention. Conference proceedings or studies without a published full text were excluded. Eighteen studies were included for full-text review, and 10 of these were included for data extraction and analysis. The risk of bias was judged to be low for seven papers, and unclear for three papers (Table 1). All included studies were categorized as prognostic level II, and all were categorized as retrospective cohort studies. Kocher et al. examined a retrospective control group and prospective intervention group.12 All studies were located in the United States8–16 with the exception of Brehin et al., which was located in France.7 Patients included across all studies were under age 18.
Figure 1. Flowchart illustrating the process of screening for inclusion in the review.
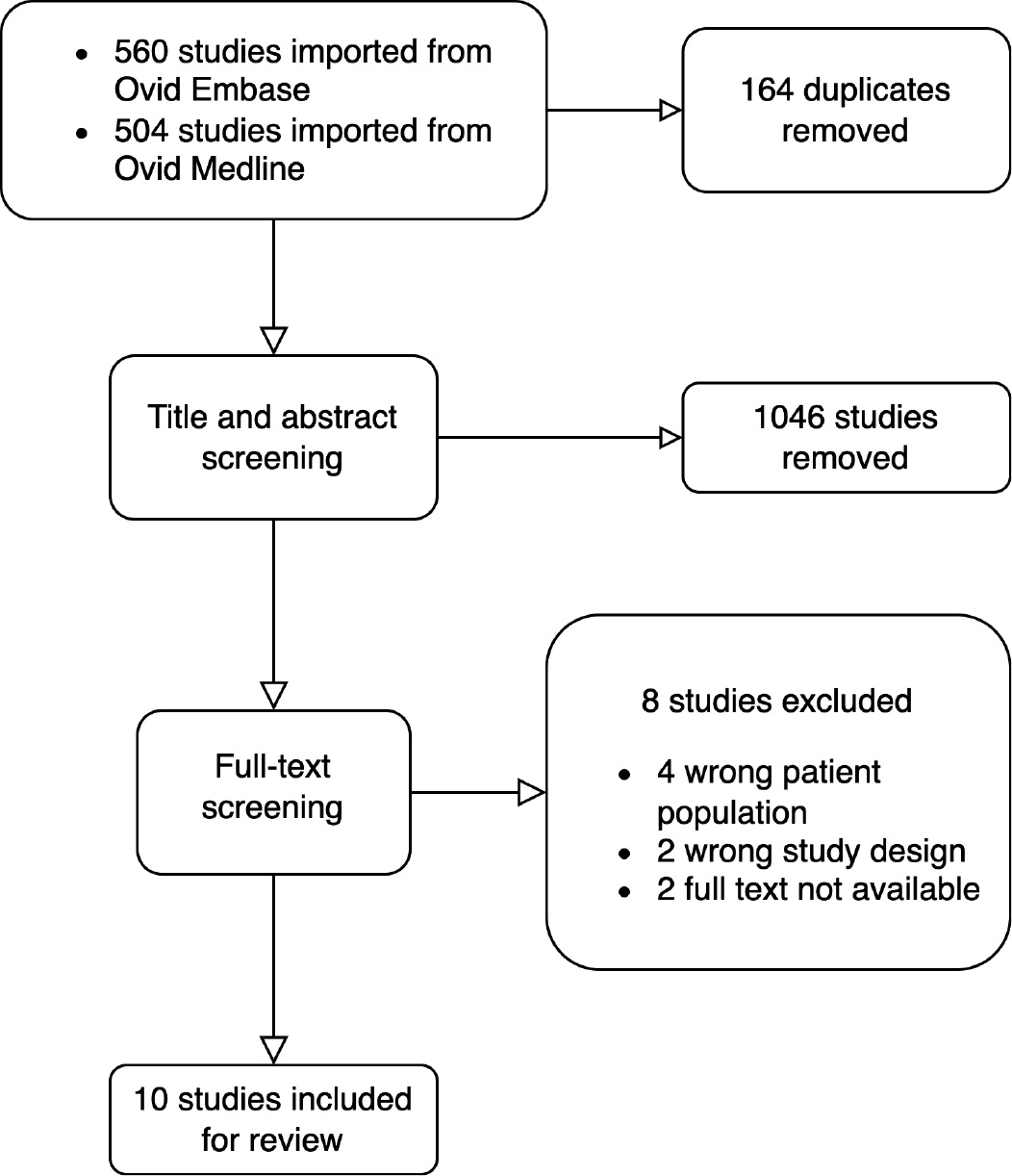
Table 1. Summaries of the Included Studies
| Author and Year | Study Size | Risk of Bias | MSKI Type(s) | Excluded Health Conditions | Laboratory Investigations | Clinical Protocols | Surgical Interventions |
|---|---|---|---|---|---|---|---|
| Bréhin et al. 20207 | 377 | Low | Osteomyelitis, osteoarthritis, acute septic arthritis, and spondylodiscitis | Chronic osteomyelitis, postoperative or post-traumatic infections, immunocompromised patients, or sickle cell anemia |
|
|
|
| Copley et al. 20138 | 271 | Low | Confirmed osteomyelitis | Any alternative diagnosis |
|
|
|
| Devine et al. 20209 | 254 | Low | Acute osteomyelitis, septic arthritis, or pyomyositis | Postoperative infection, chronic infection (i.e., >2 weeks of symptoms), recent treatment for MSKI, preceding perforating injury or trauma, central nervous system infection, or severe comorbid conditions at the time of admission (e.g., rheumatologic diagnosis, immunosuppressive condition, spina bifida, cerebral palsy) |
|
|
|
| Griswold et al. 202010 | 93 | Unclear | Osteoarticular infection | Chronic osteomyelitis, primary surgical treatment at an outside hospital, osteoarticular infection secondary to traumatic injury, immunocompromise, hemoglobinopathy, unconfirmed osteoarticular infection, or incomplete medical records |
|
|
|
| Hester et al. 202111 | 224 | Low | Acute osteomyelitis, septic arthritis, or pyomyositis | Complex chronic conditions or direct ICU admission, ≥14 days of symptoms, major trauma, postoperative infection, skull, vertebral, hand, and/or foot infection; Lyme disease, concern for necrotizing fasciitis or unusual organism, or viral myositis |
|
|
|
| Kocher et al. 200312 | 60 | Low | Septic arthritis of the hip | Major coexisting disease, postoperative infection, chronic joint infection, perforating injuries, psoriasis, polyarthritis, associated osteomyelitis, or psoas abscess |
|
|
|
| Patel et al. 201913 | 1602 | Low | Osteomyelitis, septic arthritis, pyomyositis | No documented provider concern for the prescribed MSKIs in the CMP, evaluations were conducted for other non-MSK concerns |
|
|
|
| Quick et al. 201814 | 117 | Unclear | Acute hematogenous osteomyelitis or septic arthritis | Evidence of sepsis or hemodynamic irritability, contiguous osteomyelitis (penetrating trauma or fracture), complicated osteomyelitis, history of bone or cartilage disorder, congenital or acquired bone disease, congenital or acquired immunodeficiency, diabetes, sickle cell disease, chronic sinusitis, fasciitis, synovitis, or arthropathy |
|
|
|
| Robinette et al. 201915 | 57 | Unclear | Acute hematogenous osteomyelitis | Nonhematogenous source (including previous fracture, surgery, penetrating trauma, or a pressure ulcer at or near the site of the infection), chronic osteomyelitis (i.e., antecedent symptoms lasting >2 weeks), underlying chronic illnesses, immunocompromise, multifocal infections (including septic thrombophlebitis and septic pulmonary emboli), or a protracted bacteremia (defined as positive blood culture results on ≥3 consecutive days) |
|
|
|
| Spruiell et al. 201716 | 164 | Low | Acute osteomyelitis, septic arthritis and/or pyomyositis | Postoperative or chronic infection, readmission after recent MSK treatment, perforating trauma, severe comorbid condition, initial treatment at an outside hospital, osteomyelitis of the head/orbits, or primary central nervous system infection |
|
|
|
General Clinical Care Guidelines Followed for MSKIs
The general steps taken in cases of suspected MSKIs included laboratory evaluation, pathogen identification, imaging, antibiotic therapy, and surgical interventions (Table 1). The predominant protocols followed in the included studies that improved patient outcomes included identifying the causative pathogen, faster transition from IV antibiotic administration to oral, conducting MRI earlier in the CCG, and limiting vancomycin use.
Type of Conditions Included in the CCG
The types of conditions encompassed in the CCGs included osteomyelitis, osteoarthritis, acute septic arthritis, spondylodiscitis, pyomyositis, and muscular abscess. Not all 10 studies included all conditions, but rather each study contained a variation of the conditions listed. Individual studies differed in their criteria regarding specific exclusions from participation, with some being more restrictive than others. Immunocompromised patients, co-existing disease, chronic infections, postoperative infections, and patients who already received primary care at a separate institution were frequently listed under grounds for exclusion (Table 1).
Parameters Measured in Each Study
Each study compared different outcome metrics impacted by the implementation of a CCG for MSKI patients (Table 2). Only parameters significant to care and clinical outcomes of CCGs are listed.
Table 2. A list of the Parameters Measured in Response to a CCG in Each Included Study
| Author and Year | Outcome Metrics Measured in the Study |
|---|---|
| Bréhin et al. 20207 | Bacteria identified, duration of IV antibiotic therapy, duration of total antibiotic therapy, length of stay, rate of additional surgery, frequency of MRI, relapses, and treatment failures |
| Copley et al. 20138 | Bacteria identified, duration of IV antibiotic therapy, duration of oral antibiotic therapy, duration of total antibiotic therapy, length of stay readmission rate, time to MRI, conduction of MRI, and types of antibiotics used |
| Devine et al. 20209 | Bacteria identified, duration of IV antibiotic therapy, length of stay, length of fever, readmission rates, vancomycin use, time to antibiotics, and CVC utilization rates |
| Griswold et al. 202010 | Length of stay, readmission rate, number of repeat surgeries, identification of adjacent infections, frequency of pre-operative MRI, and number of operative sites |
| Hester et al. 202111 | Bacteria identified, CVC utilization rates, vancomycin use, and hospital costs |
| Kocher et al. 200312 | Duration of IV antibiotic therapy, length of stay, symptom duration, time to surgery, readmission rates, duration of symptoms, additional surgeries, and time to oral antibiotics |
| Patel et al. 201913 | Length of stay, time to MRI, readmissions, time to antibiotics. |
| Quick et al. 201814 | Bacteria identified, duration of IV antibiotic therapy, duration of oral antibiotic therapy, length of stay, readmission rate, PICC utilization, and proportion of MRI done in 24 hours |
| Robinette et al. 201915 | Bacteria identified, length of stay, hospital costs |
| Spruiell et al. 201716 | Bacteria identified, duration of IV antibiotic therapy, duration of oral antibiotic therapy, length of stay, length of fever, time to normal CRP levels, number of surgeries, time to antibiotics, CVC use |
Length of Stay and Readmission
All studies included length of stay (LOS) as a measured outcome, and nine (90%) documented readmission rates. After CCG implementation, five studies (50%) showed a statistically significant decrease in LOS, and two (20%) showed statistically significant decrease in readmission rates. The change in mean or median length of stay across the studies ranged from a 0.4-day increase to a 5-day decrease,7–13,15,16 with only Quick et al. reporting an increase in LOS (p = 0.165).14 Griswold et al. and Spruiell et al. each found an approximately 50% decrease in readmission rate.10,16 Additionally, Griswold et al. reported a significant decrease in unplanned repeat surgeries, from 85% to 60% (p = 0.0099).10
Length of Fever
The studies conducted by DeVine et al. and Spruiell et al. found a significant decrease in length of fever, both from 63 hours to 39 hours (p = 0.04 and p = 0.012, respectively).9,16
IV Antibiotic Therapy Via Central Venous Catheterization and Oral Antibiotic Therapy and Use
Six papers studied the intervention’s impact on the duration of IV antibiotic therapy, four of those studies discussed the duration of oral antibiotic therapy, and two of them discussed the total duration of antibiotic therapy (including both IV and oral antibiotic therapy combined). Of the six studies that discussed IV antibiotic therapy, five of them found a significant decrease in the duration of IV antibiotic therapy, with pre-CCG values ranging from 7 to 22 days and post-CCG values ranging from 3 to 6 days. Copley et al. found that the duration of oral antibiotic therapy increased from 27.7 to 43.7 days (p = 0.0004),8 and Quick et al. saw an increase from 14 to 21 days (p < 0.001).14 On average, the total duration of antibiotic therapy did not change; Brehin et al. reported a decrease of 45 to 32 days (p < 0.001),7 Copley et al. saw an increase from 41.9 to 54.9 days (p = 0.04),8 while Spruiell et al. found no significant difference from 46 to 44 days after the implementation of CCGs (p = 0.5638).16 Additionally, Patel et al. measured the median time to first administering antibiotics and found a significant decrease from 9.5 to 4.9 hours (p = 0.0005).13
Five papers recorded the rate of CVC use before and after CCG implementation, and each found a significant decrease. In the cases of Devine et al., Hester et al., and Spruiell et al., CVC utilization rates in the pre-CCG group were approximately 50% and decreased to 17% or lower in the post-CCG group (p < 0.0001).9,11,16 Brehin et al. saw a decrease from 70% to 9% (p = 0.003),7 and Quick et al. from 91% to 44% (p < 0.001).14
MRI/Imaging
Four of the papers looked at the role of MRI in MSKI diagnosis and treatment protocols and measured related outcomes before and after intervention. Copley et al. found that the average time from admission to MRI was reduced from 2.47 days to 1.04 days (p = 0.0002) and the number of MRIs per child increased from 1.01 to 1.33 (p = 0.04).8 Griswold et al. showed that the frequency of preoperative MRI increased from 20% to 66% (p < 0.0001).10 Quick et al. showed that the proportion of MRIs done within 24 hours of admission increased from 67% to 83% (p = 0.077).14 Brehin et al. found that after CCG implementation, fewer bone scintigraphies were done (p < 0.001), and more MRIs were performed (p = 0.005).7 Overall, the evidence suggests that CCGs allow for earlier access to MRI, increase the rate of repeat MRI, and encourage using MRI over other imaging modalities.
Hospital Costs
Two studies examined hospital costs as a primary outcome.11,15 Hester et al. found a significant decrease in hospital costs from US$66,400 to US$53,300 (p = 0.009) after implementation of a CCG,11 and Robinette et al. showed a decrease from US$45,718 to US$32,895 but did not provide probability values.15 Hester et al. measured eight categories (professional fees, radiology, supply, pharmacy, other, laboratory, surgery, room and board) that contribute to inpatient charges, and it was noted that room and board markedly reduced the overall cost of patient stay. Robinette et al. were able to reduce hospital costs by conducting a cost-effective analysis. They examined the costs that resulted from different combinations of diagnostic interventions to determine the most cost-effective approach.15
Source Pathogen Identification Through Bacterial/Tissue Culture
Eight out of ten papers identified source pathogens. Five papers found methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) to be common bacterial strains. Five papers found Kingella kingae (K. kingae), and three found Streptococcus pyogenes (S. pyogenes) to be common bacterial strains. Robinette et al. identified Bartonella henselae (B. henselae),15 and Spruiell et al. identified Streptococcus pneumoniae (S. pneumoniae) as source pathogens.16 Brehin et al. and Spruiell et al. noted that pathogen identification leads to a decrease in the overall length of stay with no additional morbidity.7,16 Quick et al. found that bacterial identification allowed for targeted antibiotic therapy but were unable to detect a significant reduction in the length of stay or readmission rate after the implementation of the guideline.14
Discussion
The implementation of CCGs improves access to care and clinical outcomes for pediatric MSKIs. This systematic review utilized data from studies found through Ovid Embase and Ovid Medline. No other databases were searched as the resources resulting from the searches on these two databases comprehensively covered the topic being reviewed.
Length of stay is an important outcome and indicates the efficiency of hospital management. Shorter LOSs are associated with a lower risk of nosocomial infection and more efficient use of hospital resources.17 Reducing the length of stay not only alleviates the financial burden on patients but also facilitates quicker turnover of hospital beds. This, in turn, increases the hospital’s profit margin while minimizing medical expenses of treating pediatric MSKI for the patient and their family as demonstrated by the analysis of Hester et al. and Robinette et al.11,15
Consistently, we found that decreasing the duration of IV antibiotics with CCG decreases the need for CVCs, which further decreases the LOS, patient morbidity, and cost of care. Kocher et al. developed a CCG in 1995 which recommended an earlier switch to oral antibiotics.12 They found that quicker transitions to oral antibiotics were associated with shorter hospital stays, lower costs, and ease of management for the patients and families. Additionally, they noted that there was no increase in the rates of complications such as readmission, reinfection, recurrent drainage, or limitation of motion. Quick et al. and Spruiell et al. also cite evidence for benefits of earlier transition to oral antibiotics and implemented their CCG accordingly.14,16 The use of CVCs was specifically discouraged. A study by Keren et al. (2015) found that antibiotic therapy by CVC was not advantageous compared to oral delivery, because CVC use has a higher risk of severe complications, such as bloodstream infection, thromboembolism, and line breakage, and may lead to higher readmission rates.18 Similarly, the CCG implemented at Children’s Hospital Colorado in 2012 as described by Spruiell et al., also calls for CVCs to be avoided.16 Efforts are underway to minimize the use of vancomycin in the treatment of MSKIs. The CCG developed by Hester et al. at two Midwestern United States hospitals in 2016 also encouraged limiting use of CVCs and empirical vancomycin in an effort to reduce variation in providers’ approaches to antibiotic therapy.11 They tried to decrease empirical vancomycin by limiting its use to individuals that have allergies to cephalosporins, but they did not report a significant change.11
MRI plays an important role in the diagnosis and treatment of MSKIs, and implementing CCGs can provide earlier access to MRI by establishing times dedicated to imaging MSKIs. Griswold et al. found a significant decrease in repeat surgeries by implementing a CCG which called for all patients with suspected osteoarticular infection to undergo preoperative MRI.10 To achieve this, an MRI time slot was reserved for MSKI patients immediately prior to their scheduled OR time. Surgeons and radiographers would review the results of the MRI and decide whether surgical intervention was required. If so, a surgical plan could be composed, and the optimal operation could be done immediately after the MRI. Similarly, the design of the CCG implemented by Copley et al. was to reserve a daily MRI and OR time slot early in the morning for MSKI patients.8 This routine of dedicated time slots proved to be more practical than requesting immediate MRI and ensured that patients would not wait longer than the time until the next day’s MRI time slot. Additionally, patients could avoid having multiple sedations since the same sedation is used for both MRI and OR. The improved efficiency in access to MRI in the early stage of hospitalization leads to sooner surgical intervention and shorter lengths of stay.2 The same practice was found in the CCGs implemented by Hester et al. and Quick et al.11,14 Future research on the impacts of early MRI use for MSKI patients may demonstrate to be significant in patient and clinical outcomes.
A limitation of this study is that all included papers followed the format of comparing cohorts from different time periods (before and after guideline implementation, and several years after implementation in DeVine et. al), leading to an inherent bias due to potential confounding factors across time periods. This study is also limited by its heterogenous study batch, seeing that the cohorts of each paper in this study, although similar, were defined by unique inclusion and exclusion criteria. Since the data included in this systematic review are extracted from existing studies, the quality of this paper is limited to the biases and quality of the original articles. This systematic review did not carry out meta-analyses due to the heterogeneity of the data, as it is a quantitative approach that combines and analyzes numerical data from multiple studies. Instead, this paper focused on qualitative analyses of the extracted data, examining and interpreting both numerical and non-numerical data. Future improvements and contribution to the research question at hand could be strengthened by more prospective data and further analysis.
An optimal clinical care guideline will vary depending on an institution’s resources, but several important variables were noted in this review that should be strongly considered for inclusion. Initial diagnostic evaluation of a previously healthy pediatric patient with signs and symptoms suggestive of an acute MSKI should include a CBC with differential, CRP (+/- ESR), blood cultures, and plain film X-ray of the affected area. A patient with concerning signs of sepsis should then be started immediately on broad spectrum IV antibiotic coverage, with a consideration to minimize vancomycin use when possible. Both orthopaedics and general pediatrics consults are appropriate, and consultation with the infectious disease team is recommended. An MRI should be obtained as soon as possible, ideally within 24 hours of presentation. This imaging would then be used to help determine whether the patient requires operative intervention, and in cases where surgery is required, would help ensure the infection is appropriately addressed to avoid unexpected return to the OR. Coordinating anaesthesia, radiology, and surgical teams to conduct an MRI immediately before a scheduled surgery would further optimize outcomes. This would help to limit the number of sedatives used in pediatric MSKI patients, as they could be immediately transferred from the MRI to the operation room under the same anaesthesia (if surgery is deemed necessary). There are risks associated with sedating young patients multiple times within a short period; therefore, achieving this coordination would be beneficial. Antibiotics should be tailored to identified organisms in blood and/or infectious site cultures. Ongoing evaluation includes a measurement of CRP +/- CBC every 48 hours. Step-down to oral antibiotics and avoidance of CVC use should be priorities, with step-down criteria based on clinical improvement, resolution of fever, and improved CRP. Ideally, a CCG for MSKI should be made as a collaborative effort between the services involved, including orthopaedics, general pediatrics, radiology, emergency medicine, and infectious diseases. Although a coordinated effort can be time-consuming, this review shows that a thoughtful, institution-appropriate CCG for MSKI has the potential to decrease LOS and readmission rates, limit CVC use, and lower hospital costs.
Disclaimer
No funding was received. The authors report no conflicts of interest related to this manuscript.
Additional Links
- 2015 POSNA Annual Meeting Paper: Prevalence and Complications of Musculoskeletal Infections in Adolescents: A Result of Delay in Diagnosis?
- 2015 POSNA Annual Meeting ePoster: Is it Worth the Effort? The Outcomes of a Multidisciplinary Clinical Care Guideline for Acute Pediatric Musculoskeletal Infections
- POSNAcademy: Musculoskeletal Infection Hijacking the Acute Phase Response
References
- Funk SS, Copley LAB. Acute hematogenous osteomyelitis in children: pathogenesis, diagnosis, and treatment. Orthop Clin North Am. 2017;48(2):199-208.
- Copley LAB. Pediatric musculoskeletal infection: trends and antibiotic recommendations. J Am Acad Orthop Surg. 2009;17(10):618-626.
- Neuman MI, Hall M, Hersh AL, et al. Influence of hospital guidelines on management of children hospitalized with pneumonia. Pediatrics. 2012;130(5):e823-e830.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285-292.
- Parker S, Spruiell M, Pyle L, et al. Pediatric acute musculoskeletal infection clinical care guideline improves outcomes. Open Forum Infect Dis. 2015;2(suppl_1):1530.
- Tjosvold L, Campbell SM, Dorgan M. Filter to Retrieve Pediatric Articles in the OVID Medline Database. John W. Scott Health Sciences Library, University of Alberta. Rev. September 14, 2020. Available at: https://docs.google.com/document/d/1Q3MLfUolWe9q33JdAIzmVK0vi_ieC2Z60e9QvzTgkU8/edit.
- Copley LAB, Kinsler MA, Gheen T, et al. The impact of evidence-based clinical practice guidelines applied by a multidisciplinary team for the care of children with osteomyelitis. J Bone Joint Surg Am. 2013;95(8):686-693.
- DeVine MN, MacBrayne CE, Williams MC, et al. Long-term impact of a clinical care guideline for pediatric acute musculoskeletal infections: are improved outcomes sustainable? Hosp Pediatr. 2020;10(12):1107-1113.
- Kocher MS, Mandiga RB, Murphy JMR, et al. A clinical practice guideline for treatment of septic arthritis in children: efficacy in improving process of care and effect on outcome of septic arthritis of the hip. J Bone Joint Surg Am. 2003;85(6):994-999.
- Bréhin C, Claudet I, Dubois D, et al. Assessing the management of pediatric bone and joint infections according to French guidelines. Med Mal Infect. 2020;50(6):515-519.
- Griswold BG, Sheppard E, Pitts C, et al. The introduction of a preoperative MRI protocol significantly reduces unplanned return to the operating room in the treatment of pediatric osteoarticular infections. J Pediatr Orthop. 2020;40(2):97-102.
- Hester GZ, Nickel AJ, Watson D, et al. Improving care and outcomes for pediatric musculoskeletal infections. Pediatrics. 2021;147(2):e20200118.
- Patel L, Michael J, Allen N, et al. Experience with a care process model in the evaluation of pediatric musculoskeletal infections in a pediatric emergency department. Pediatr Emerg Care. 2019;35:605-610.
- Quick RD, Williams J, Fernandez M, et al. Improved diagnosis and treatment of bone and joint infections using an evidence-based treatment guideline. J Pediatr Orthop. 2018;38(6):e354-e359.
- Robinette ED, Brower L, Schaffzin JK, et al. Use of a clinical care algorithm to improve care for children with hematogenous osteomyelitis. Pediatrics. 2019;143(1):e20180387.
- Spruiell MD, Searns JB, Heare TC, et al. Clinical care guideline for improving pediatric acute musculoskeletal infection outcomes. J Pediatric Infect Dis Soc. 2017;6(3):e86-e93.
- Baek H, Cho M, Kim S, et al. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One. 2018;13(4):e0195901.
- Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015;169(2):120-128.
