Current Concept Review
Estimating Skeletal Age in Children: A Comprehensive Anatomic Approach
1Drexel University College of Medicine, Philadelphia, PA; 2Boston Children’s Hospital, Orthopedic Center, Boston, MA; 3Rady Children’s Hospital, San Diego, CA; 4University of California–San Diego School of Medicine, San Diego, CA; 5Harvard Medical School, Boston, MA
Correspondence: Benton E. Heyworth, MD, Boston Children’s Hospital, 300 Longwood Ave., Hunnewell 217, Boston, MA 02115. E-mail: [email protected]
Received: February 22, 2022; Accepted: March 7, 2022, Published: May 1, 2022
DOI: 10.55275/JPOSNA-2022-0027
Volume 4, Number 2, May 2022
Abstract:
While arguably the most widely utilized method of determining skeletal age is the Greulich and Pyle method (GPM), it unfortunately has many shortcomings. The GPM may not lend itself to widespread use or optimized learning in the modern era of orthopaedics as the method relies on limited population metrics and is dependent on a voluminous hard-copy radiographic atlas. An expanding body of research has emerged over the past 2 decades, attempting to provide simpler yet effective tools to determine skeletal age. In this review, we compare and present the spectrum of current validated bone age determination methods for six commonly imaged anatomic locations. All methods are presented visually in table format, with detailed graphic design, quantitative approximations to generate relative consistency between systems, and age-based color-coding for ease of memorization and use. The purpose of this review is multifaceted: to enable better incorporation of skeletal age considerations into daily practice by caregivers in many disciplines, to enhance awareness regarding the full array of tools that may spare young patients additional and unnecessary radiation, and to serve as a springboard for a mobile application to be utilized in any clinical setting by anyone with a mobile device.
Key Concepts:
- Skeletal age is a unique patient variable that must be taken into consideration in order to provide optimal care in pediatric orthopaedic surgery.
- While the Greulich and Pyle method (GPM) remains the most commonly utilized approach, the practical application of the method has many limitations in the modern era.
- It has previously been demonstrated that modern “shorthand” approaches to the detailed historic methods may be just as effective, while also being significantly more expeditious, efficient, and comprehensible for use.
- A single reference guide that provides an anatomically comprehensive overview to the principles associated with skeletal age estimation, bringing an array of current validated bone age determination methods together, with easily referenced visual presentations and elements of consistency throughout the systems, may be of great utility to pediatric caregivers.
Introduction
The skeletal maturity of a child is a critical consideration when deciding how best to manage many pediatric conditions.1,2 Because the chronologic age of a child or adolescent represents only a rough estimate of that individual’s skeletal development, pediatric orthopaedic surgeons rely on the enhanced accuracy and precision of skeletal maturity assessments to guide decision-making regarding ideal timing and technique for a number of procedures.3,4 Currently, one of the most widely accepted gold standard techniques for bone age determination is the Greulich and Pyle method (GPM), but this can only be utilized in the presence of a hard copy of the voluminous Radiographic Atlas of the Hand and Wrist, published in 1959.5 Obviously, such a cumbersome approach has a number of drawbacks in the modern era, which represents a vastly different setting of orthopaedic knowledge and practice from that of the 1960s, when the atlas began to see routine use by some clinicians.
Due to the overall complexity of the GPM, as well as its dependence on a large hard-copy atlas, it does not tend to be the subject of formal training outside of a radiology residency or musculoskeletal radiology fellowship. Such circumstances lead orthopaedic surgeons, general pediatricians, pediatric endocrinologists, and other practitioners to be dependent on radiologists for bone age readings.3 Additionally, the GPM often requires a child to receive what is most likely an additional x-ray of the left hand. Not only does this increase cumulative radiation exposure and burden to the patient and family but it adds to the complexity and costs of healthcare visits amidst an era aiming to cut costs and enhance patient safety and satisfaction.4,6
In attempt to compensate for these limitations, an expanding body of research has emerged in the last 2 decades designed to propose and validate alternative methods to the GPM. For example, the Shorthand Bone Age (SBA) Method,3 published in 2013, arose as a derivation of the GPM atlas itself. In this simplified approach, which employs just one graphic for each sex, the SBA reliability study identified the single most pertinent radiographic characteristic at each skeletal age group, each of which was shown to be as effective as the exhaustive constellation of findings previously described for these ages in the GPM. As a result, SBA parameters can be memorized by its users and trainees, or the single graphic can be posted in one’s clinic or saved on a mobile device. Moreover, other anatomic regions of the skeletal system have also been the subject of studies designed to estimate bone age. Indeed, there are published bone age determination methods for the majority of the most commonly imaged body parts, each of which has been assessed regarding intra- and inter-observer reliability. However, given the large number of publications describing these methods across many different journals over a relatively long time period, it has been difficult to date to incorporate such new methods or a comprehensive approach to skeletal age estimation into daily practice as well as decide which methods truly merit doing so. This current concept review aims to present what may be considered the existing gold standard methods for skeletal age determination from either plain radiographs or magnetic resonance imaging (MRI) at each of six different body parts: hand/wrist, elbow, shoulder/proximal humerus, hip/pelvis, knee, and foot/ankle. The purpose of this review is to provide all pediatric providers with an anatomically comprehensive and simple reference guide that estimates the skeletal age of a growing child regardless of the form of imaging that might have been recently obtained. In addition, the following tool is also designed to contribute to trainee education, enhance clinical research that might rely upon skeletal age estimation, and optimize the practice of pediatric orthopaedic surgery on a daily basis.
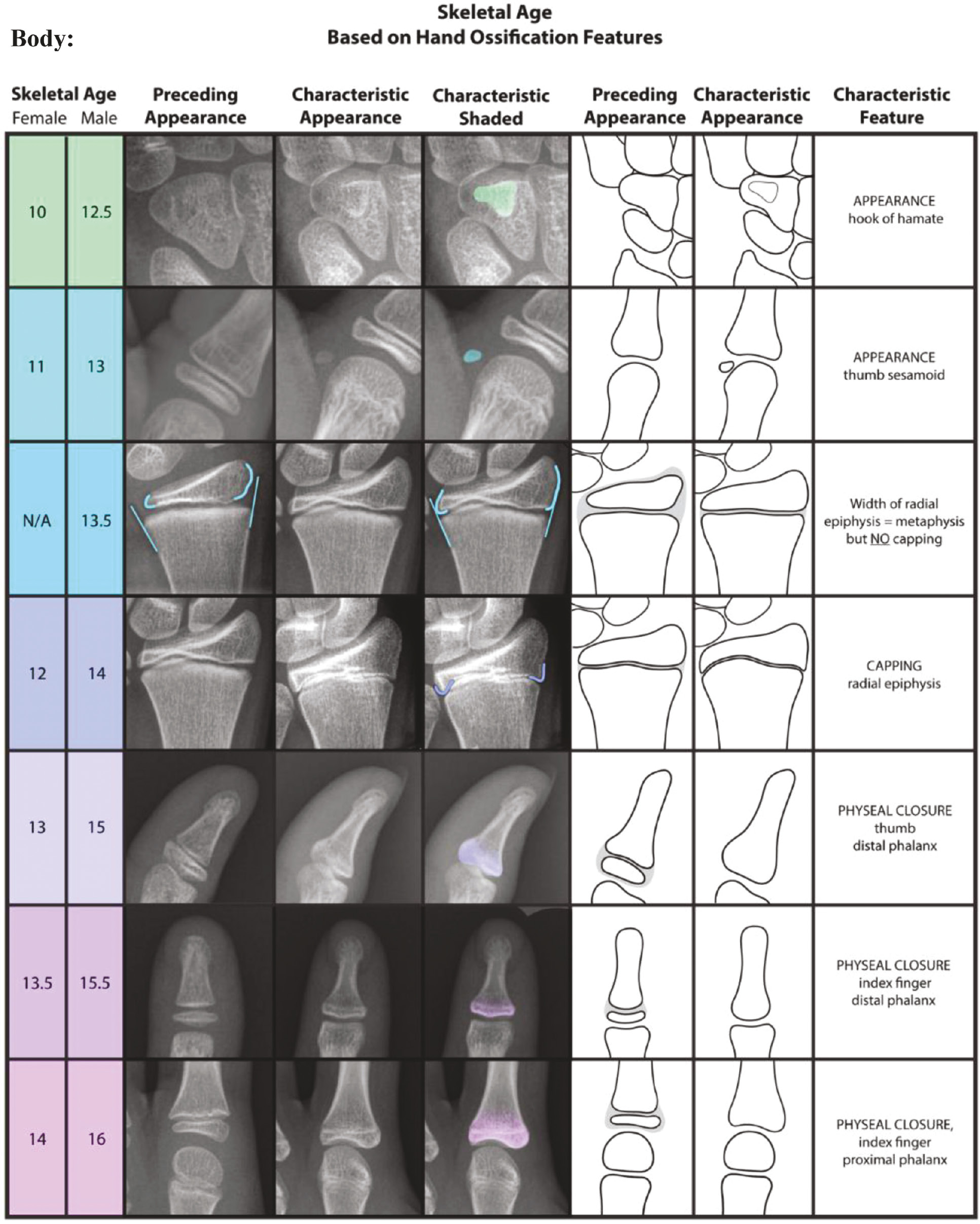
The SBA greatly simplifies the GPM itself by selecting the single most characteristic feature of skeletal maturity for each age group (Figure 1) outlined in the Radiographic Atlas of Skeletal Development of the Hand and Wrist. The advantages of the SBA over the GPM are ease of use, memorability, transferability, and teachability, all in the setting of comparable reliability and effectiveness. However, several other well-recognized methods of determining skeletal maturity via the hand/wrist also exist, including the Tanner-Whitehouse III (TW3) method7 and its simplification by Sanders et al.8 The TW3 method allows one to determine bone age from a “RUS (radius, ulna, small bones of the hand) score” that is calculated by assigning a point value to radiographic features in the distal radial and ulnar epiphyses and the metacarpal and phalangeal epiphyses of the first, third, and fifth digits. However, the RUS score can only be determined by referencing a similarly voluminous radiographic atlas, giving the TW3 method the same limitations as those of the GPM. Sanders’ simplified method sought to address these limitations by creating an eight-stage system based off the specific criteria that contribute to the “DSA (digital skeletal age) score,” a component of the RUS score. Their motivation for creating this system was based on their prior finding that the DSA correlated highly with the timing of curve progression in girls with adolescent idiopathic scoliosis (AIS).9 However, the system may be limited by the fact that the study population for the method’s development and validity testing was 22 girls with AIS. Moreover, the primary aim for this method was to predict curve progression rather than discrete determination of skeletal age. Thus, the system may not be reliably applicable to the general pediatric and adolescent population.
Figure 1. The Shorthand Bone Age Assessment.3
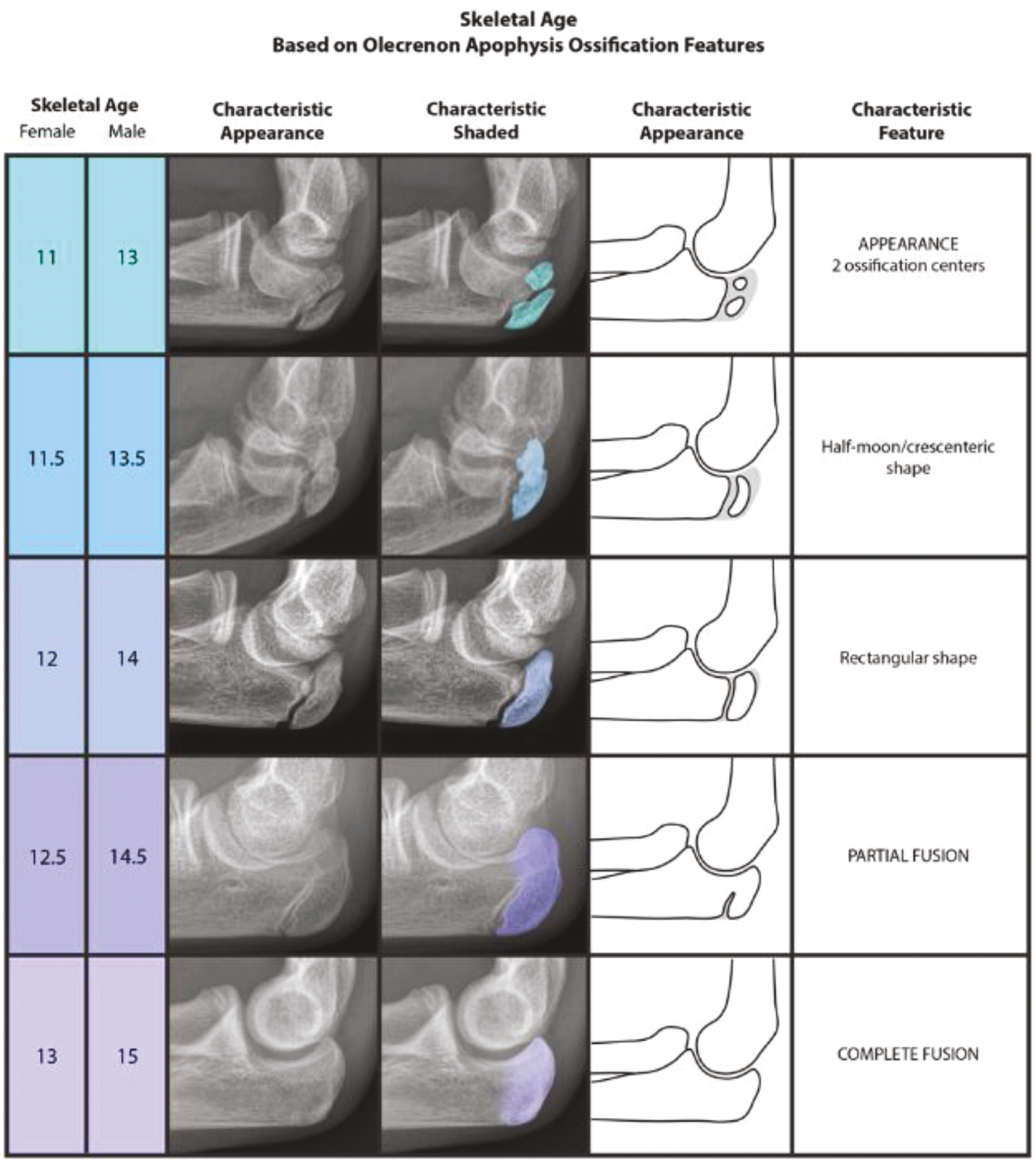
The original Sauvegrain method was published in 1962 and focused on the sequential development of each of the four ossification centers at the elbow: lateral condyle and epicondyle, trochlea, olecranon apophysis, and proximal radial epiphysis. Each ossification center has its own numeric scoring system based on the presence of certain skeletal features, and the four sub-scores are then summed to yield a total score. This final score, when plugged into the y-axis of the appropriate graph as illustrated in Sauvegrain’s original manuscript, will give the approximate skeletal age of the patient. In 2005, Dimeglio et al. sought to expand and improve upon the Sauvegrain method by updating the original method and proposing a new, simplified method based on a single ossification center (the olecranon apophyis). The update to the original method involved adding an additional morphologic stage to the sequences for the trochlea, olecranon, and proximal radial epiphysis as well as recalibrating the graphs to be linear. Previously, the graphs were sigmoidal in shape with a very steep center, making them quite difficult to use.
Dimeglio’s method represents a uniquely usable alternative to the earlier approaches because it does not require a reference graph, a unique numeric scoring system, or evaluation of four separate anatomic regions of the elbow. Nevertheless, it is necessary to note the very narrow window of time for which the method can be used. This may be less of a weakness of the method however and more of a weakness in utilizing the elbow as a standalone for skeletal age determination, as bony development at the elbow occurs very rapidly. Interestingly, the authors themselves articulate that in the instances in which they need a truly accurate bone age as a major component of surgical planning, they tend to use their update to the full Sauvegrain method (vs. only the olecranon system, Figure 2) in combination with the GPM. Nevertheless, the olecranon apophyseal method of determining skeletal maturity has considerable advantages in that it may be viewable in virtually every set of elbow and forearm radiographs (due to the reliance upon just a lateral view), and it has great simplicity and learnability.
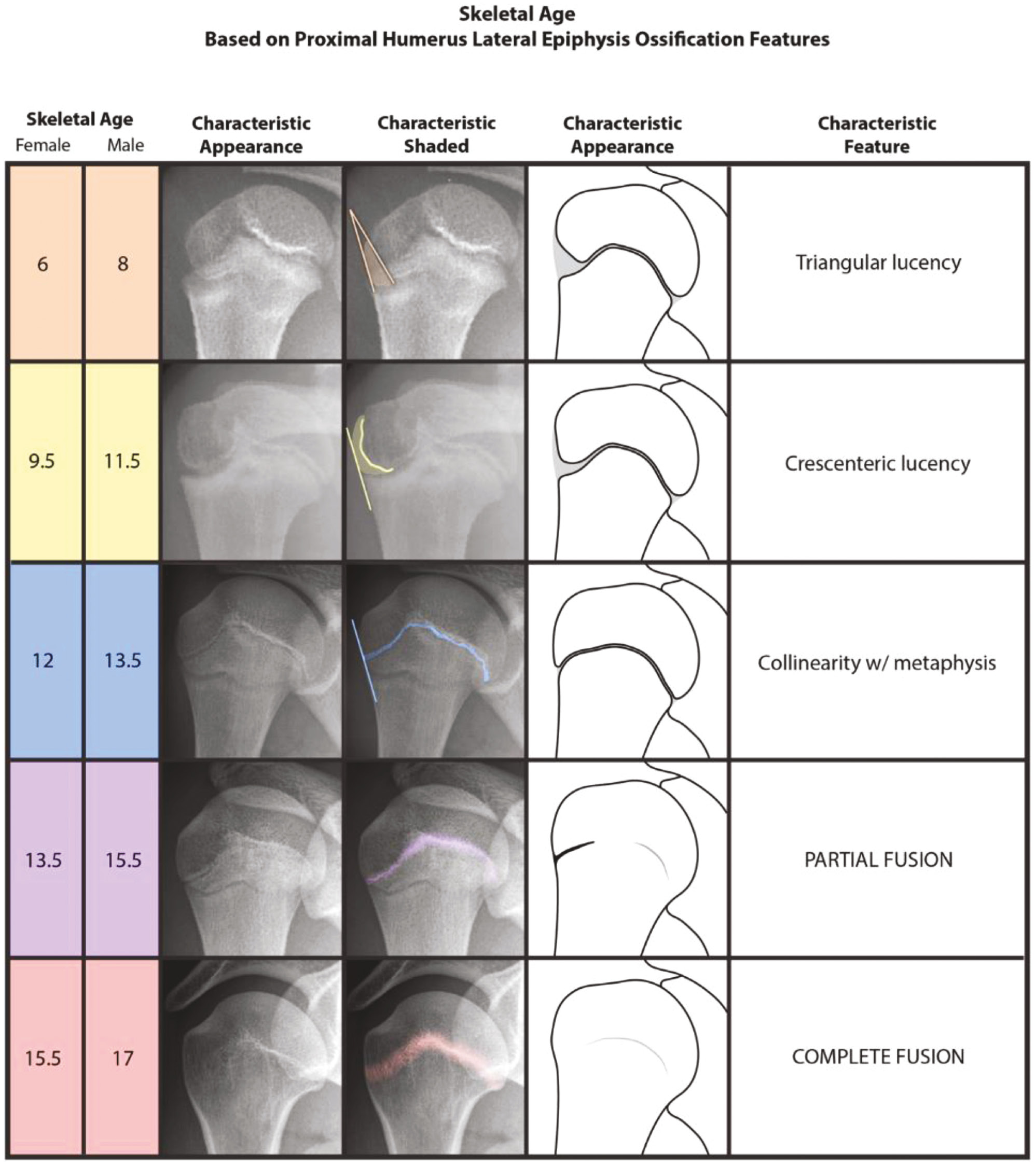
The Humeral Head Ossification System (HHOS) is the first and only validated skeletal age determination method for this anatomic region. Of note, the 2018 paper by Li et al. did not present the chronologic ages seen in Figure 3. Instead, they referenced each stage in relation to the adjusted peak growth age (PGA) or the age at which a child undergoes peak height velocity (PHV). Estimations of the chronologic ages for the current study (Figure 3) were derived through calculations by adding the adjusted PGAs to the mean PGA of either the boys or girls as appropriate from the Brush Inquiry,1 which was the patient population used by Li et al. in the development of the HHOS. Calculated chronologic ages were also rounded to the nearest 0.5 year for sake of simplicity and consistency across all methods presented here.
Figure 3. The Humeral Head Ossification System (HHOS).12
Despite the above method being the only validated skeletal age determination tool for the shoulder region, there have been recent investigations into the development of secondary ossification centers visible on MRI at both the glenoid13 and the humeral head.14 In their glenoid ossification study, Sidharthan et al. characterized three separate secondary ossification centers: anterior glenoid rim, superior glenoid rim, and coracoid. They sought to classify the development at each of these centers into one of four stages: pre-ossification, cartilage anlage, ossification, or fusion. Their motive for describing these secondary ossification centers within this four-stage system was to eliminate apparent confusion surrounding the appearance of normal anatomic glenoid development versus true pathology, most notably bony Bankart lesions. Similarly, Kelly et al. described a four-stage system based on humeral head and greater tuberosity ossification features visible on MRI with the intention of better differentiating normal anatomic development from Hill-Sachs lesions. Both of these developmental sequences were designed to be descriptive in nature; neither was intended to become a new classification system used by others for the determination of skeletal age. Nevertheless, caregivers should be aware of the stages of ossification at both the glenoid and the humeral head visible on MRI as described by Sidharthan et al. and Kelly et al., respectively, especially when evaluating for signs of shoulder instability in teenage and adolescent patients. Not infrequently, adult sports medicine and shoulder surgeons will treat adolescents or even pre-adolescents for shoulder instability or labral pathology. In these cases, surgeons may have concerns regarding iatrogenic growth disturbance with interventions on the glenoid rim. Even the Latarjet procedure may be indicated for an adolescent with a large bony Bankart lesion or attritional bone loss due to recurrent instability episodes.15 However, there are no studies to suggest that growth disturbances of the glenoid can arise from such procedures or techniques, and given the established understanding of glenoid and humeral head ossification,12–14 sequential imaging or technical modifications should not be warranted.
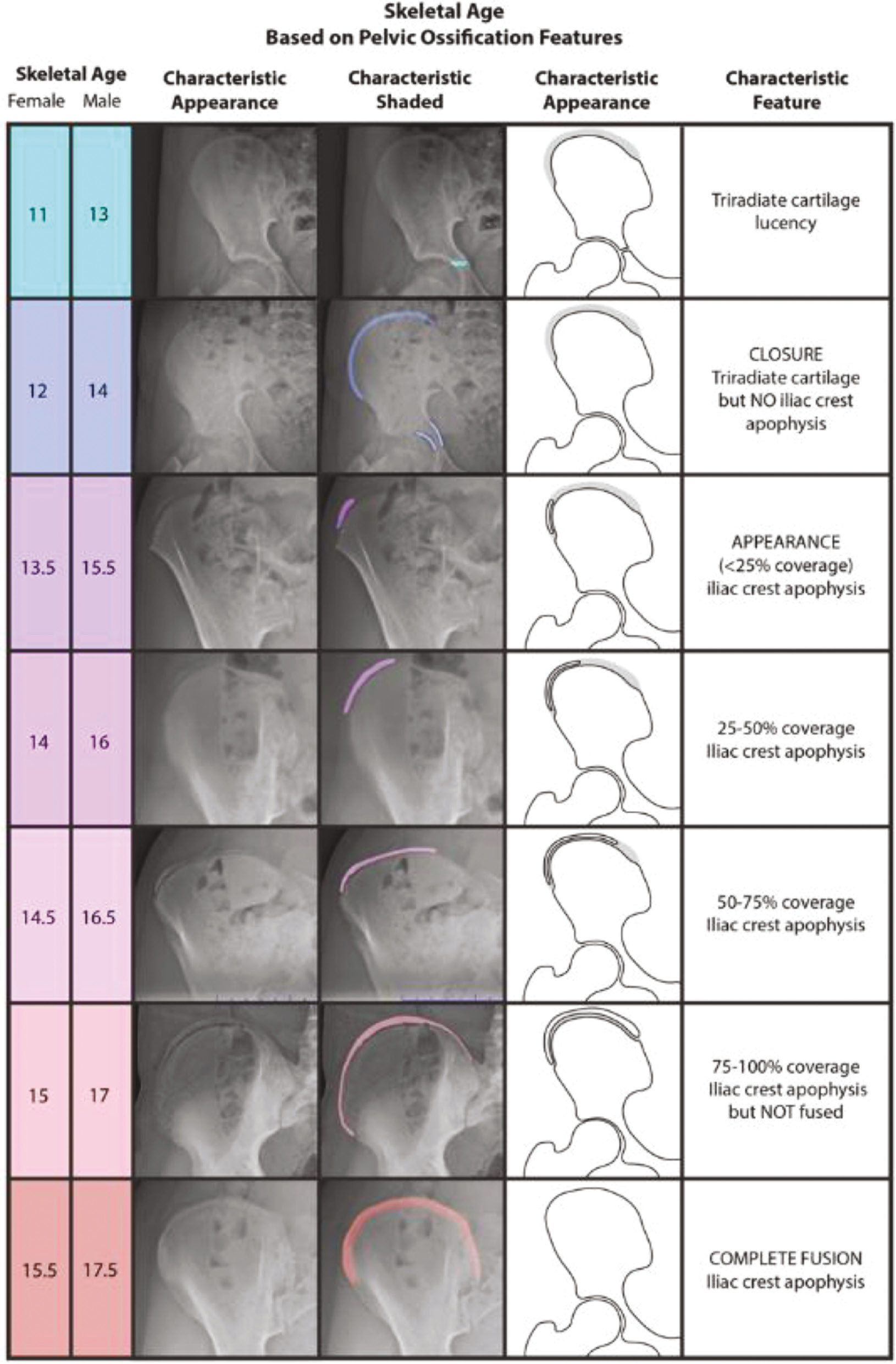
The original Risser classification system was published in 1958, predating even the GPM. It has been a major reference for skeletal age determination since its inception, especially by spine surgeons and related caregivers who manage AIS. However, despite the Risser system withstanding the test of time, it has received a great deal of criticism over the years. Many have questioned its validity and reliability in predicting skeletal age, trunk growth, and progression of AIS.16 The predominant limitation of the original Risser system was that its first stage (Risser 0) remained unchanged throughout much of a child’s pubertal growth spurt. As a result, Dimeglio proposed that Risser 0 be separated into two sub-stages based on the closure of the triradiate cartilage (TRC) of the hip.2,17 Indeed, Troy et al. incorporated this modification into their “Risser+” system,18 which also combined both the North American (NA) and European (EU) versions of the Risser system into a single new comprehensive method. While the Risser+ system holds great potential for eliminating the inconsistency between the NA and EU Risser systems and better characterizing skeletal maturity around the time of PHV, to date the system has only been validated in terms of inter- and intra-rater reliability and agreement; it has not been studied for the purpose of correlating each of its stages to a distinct level of skeletal maturity. Therefore, we recommend the original Risser system with the split of Risser 0 into TRC open and TRC closed (Figure 4). However, we acknowledge that there may be limitations to this system and its relation to skeletal age, particularly with respect to Risser 2–4.
Figure 4. The Risser system,19 with Risser 0 split into two separate stages based on the closure of the triradiate cartilage (TRC) of the hip.2,17,18
The shorthand knee MRI tool,6 developed within the past several years, represents the validated application of the knee MRI bone age atlas published by Pennock et al. in 2018.20 This atlas identified sequences of reproducible radiographic features for males and females seen on knee MRI for the femur, tibia, fibula, and patella, all in both coronal and sagittal views. Following the same trend as many other prior methods, the shorthand MRI assessment sought to simplify the knee MRI atlas by focusing on the single most distinct and reliable feature visible for each age group (Figure 5).
Figure 5. The Shorthand Knee MRI Bone Age Assessment Tool.6
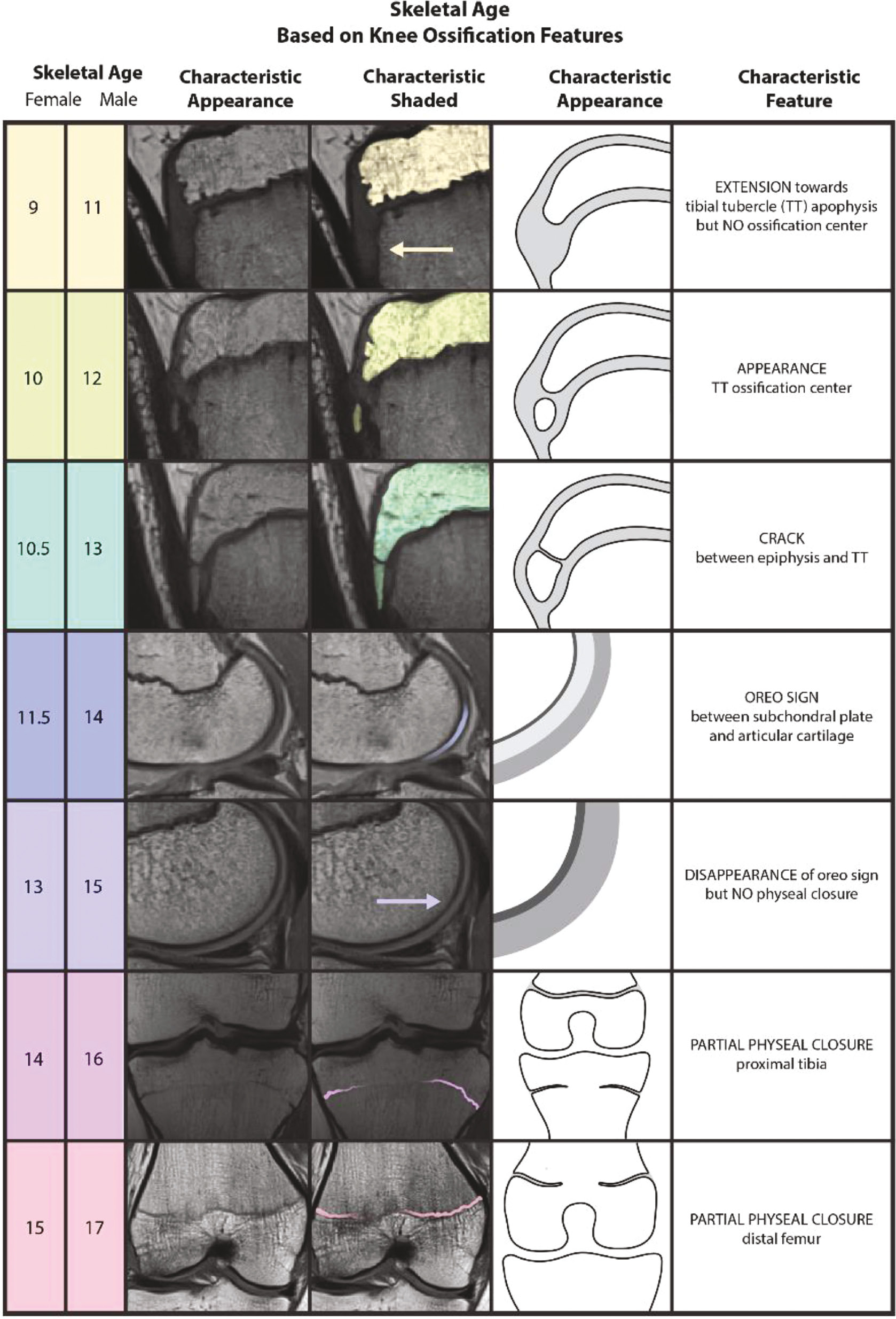
Interestingly, Meza et al. also developed a shorthand approach to bone age21 using Pennock et al.’s 2018 atlas.20 They presented eight sequential milestones for both males and females, which they consider to be the most notable radiographic feature across all four anatomic progressions at each age group. However, this method’s major limitation, when compared to that of Politzer et al., is that the proposed sequence of features is not identical between males and females. For example, two of their eight proposed milestones for males are features of patellar ossification, which are absent in the female milestones, as described in the system. In addition, for features or stages that are the same across sexes, they do not necessarily appear in the same sequential order. For instance, complete ossification of the tibial tubercle apophysis is the third milestone for females and the sixth milestone for males. The shorthand method of Politzer et al. has a completely identical sequence of features or ossification ‘events’ for both males and females, providing enhanced relative simplicity and learnability.
Another recent bone age method utilizing knee imaging worth noting is that developed by Dekhne et al.4 The authors proposed a four-stage method based on features of the tibial apophysis that can be seen on lateral view plain radiographs. This thoughtfully simple system can be of great utility through use of lateral view plain radiographs, which are almost always readily available with patients for whom ACL reconstruction is being considered. While it is not meant to be a standalone method for bone age determination, it does provide a guide of the essential stages of growth that tend to dictate technique-based treatment decisions, such as ‘physeal-sparing’ techniques versus ‘physeal respecting’ techniques. Therefore, the isolated tibial tubercle radiographic appearance represents a useful and accurate data point in the context of the larger clinical picture, helping pediatric sports medicine surgeons avoid additional imaging when determining ACL reconstruction options for a given child or adolescent.
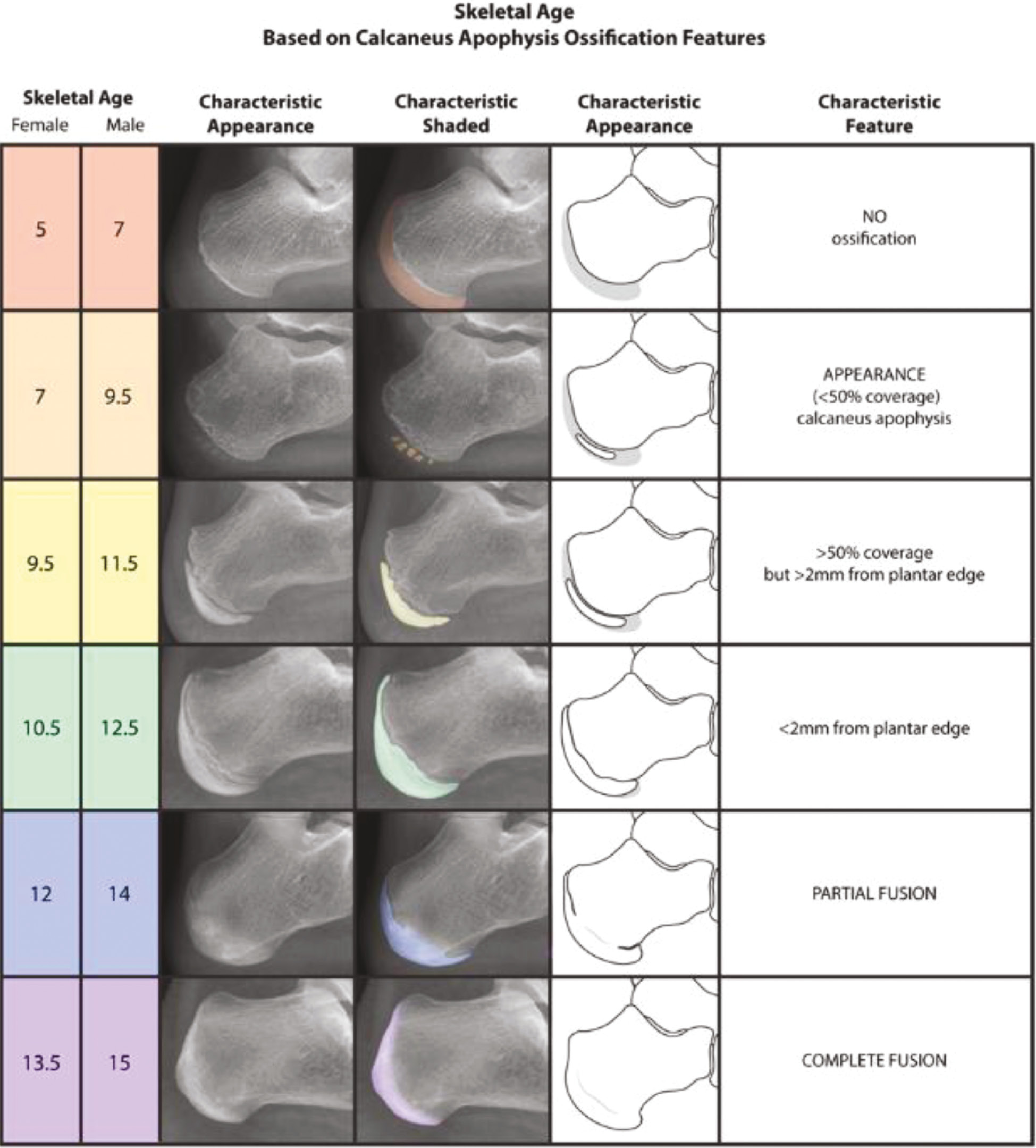
A number of the same authors who developed the HHOS12 (Figure 3) also contributed to the development of the calcaneal apophysis ossification system.22 Of note, the ages in our modified version of the calcaneal system, as detailed in Figure 6, were rounded to the nearest 0.5 year from those presented in the original manuscript. Nicholson et al.22 reported calculating the skeletal ages from the adjusted PGA and were specific to the hundredth decimal point. For the sake of user or trainee education and memorization as well as consistency across different systems, the rounding was utilized for ease of use in the setting of the more comprehensive presentation of multiple bone age estimates.
Figure 6. The calcaneal apophysis ossification system.22
Another method of bone age determination in the foot is the metaphysis, epiphysis hook skeletal assessment (MEKKA),23 also developed by the same core group of authors as above. MEKKA is another six-stage sequence of skeletal maturation, but its focus is the progressive ossification of the epiphyses of the proximal and distal phalanges of the foot. While this system is nearly identical to the calcaneal apophysis system in methodology, its main limitation lies in the physical acquisition of the imaging. As stated by the authors, the appreciation of the physes necessary for this method requires plain radiographs to be taken nearly perpendicular to the physeal plate. While possible, obtaining such images in a child is imaginably more difficult than obtaining a lateral view of the calcaneus sufficient for appreciation of its apophyseal ossification features. Importantly, the lateral view of the calcaneus has the additional advantage of being present in the standard views of both dedicated foot and ankle radiographs. Consequently, as the authors23 thoughtfully note, the MEKKA may best serve as a complementary tool to the calcaneal method when the necessary imaging is readily available, but it is not meant to supersede the calcaneal method.
Summary
The combination of six individual bone age assessment tools, each for a different commonly imaged anatomic location and modified to have similar categories, feature identification tools, and general rationale, may collectively serve as a single, simplified skeletal maturity reference guide. The tables and images presented here should allow practitioners from any discipline to make a reasonable and accurate estimate of skeletal age for most patients, regardless of which anatomic set of imaging the patient may require or with which they present. Importantly, the methods collectively span most of the critical stages of both prepubertal and pubertal growth. In addition, the attempted consistency of formatting and the graphic design features were devised to contribute to healthcare trainee education and to enhance the integration of skeletal age into daily practice in pediatric orthopaedic surgery.
Limitations of each individual skeletal age determination method were discussed in the body of the text. One potential limitation of this comprehensive review as a whole is the inability to account for the differences in patient populations used for development of each anatomic method, given that each was developed as part of independent investigations on different sub-populations. Additionally, several of the methods12,22 were developed using sub-samples of the Brush Inquiry cohort, which was comprised of predominantly white children (92.2% white, 7.7% back) from upper-class socioeconomic backgrounds growing up in the greater Cleveland area during the 1930s.24 Several other presented methods were developed using more modern and diverse urban pediatric populations.3,6 There have been recent efforts to specifically address the shortcomings of the Brush cohort in terms of diversity.25 Greater work in this area is still desperately needed to ensure all methods optimize their reliability when applied to the broader contemporary American pediatric and adolescent population.
Nevertheless, as a comprehensive resource of multiple validated skeletal age determination methods, this resource will hopefully serve well pediatric orthopaedic caregivers, their trainees, colleagues, and counterparts across an array of related disciplines and subspecialties. Moving forward, this resource should be of great utility in enhancing delivery of the safest and most effective care possible for children with musculoskeletal disease and injury.
Additional Links
- POSNAcademy: Lower Extremity Growth Arrest, How to Stay Out of Trouble, Amy L. McIntosh, MD—https://bit.ly/3IDeBL0
- OrthoKids: Leg Length Discrepancy—https://bit.ly/3Jzn6bb
Disclaimer
No funding was received. The authors have no conflicts of interest to disclose.
References
- Sanders JO, Qiu X, Lu X, et al. the uniform pattern of growth and skeletal maturation during the human adolescent growth spurt. Sci Rep. 2017 Dec 1;7(1):16705.
- Dimeglio A. Growth in pediatric orthopaedics. J Pediatr Orthop. 2001 Jul-Aug;21(4):549–555.
- Heyworth BE, Osei DA, Fabricant PD, et al. The shorthand bone age assessment: a simpler alternative to current methods. J Pediatr Orthop. 2013 Jul-Aug;33(5):569-574.
- Dekhne MS, Kocher ID, Hussain ZB, et al. Tibial tubercle apophyseal stage to determine skeletal age in pediatric patients undergoing ACL Reconstruction: a validation and reliability study. Orthop J Sports Med. 2021 Sep;9(9):23259671211036897.
- Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford, CA: Stanford University Press; 1959.
- Politzer CS, Bomar JD, Pehlivan HC, et al. Creation and validation of a shorthand magnetic resonance imaging bone age assessment tool of the knee as an alternative skeletal maturity assessment. Am J Sports Med. 2021 Sep;49(11):2955-2959.
- Tanner JM. Assessment of Skeletal Maturity and Prediction of Adult Height. 3rd ed. London: W.B. Saunders; 2001.
- Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008 Mar;90(3):540-553.
- Sanders JO, Browne RH, McConnell SJ, et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am. 2007 Jan;89(1):64-73.
- Dimeglio A, Charles YP, Daures JP, et al. Accuracy of the Sauvegrain method in determining skeletal age during puberty. J Bone Joint Surg Am. 2005 Aug;87(8):1689-1696.
- Sauvegrain J, Nahum H, Bronstein H. [Study of bone maturation of the elbow]. Ann Radiol (Paris). 1962;5:542-550.
- Li DT, Cui JJ, DeVries S, et al. Humeral head ossification predicts peak height velocity timing and percentage of growth remaining in children. J Pediatr Orthop. 2018 Oct;38(9):e546-e550.
- Sidharthan S, Greditzer HGt, Heath MR, et al. Normal glenoid ossification in pediatric and adolescent shoulders mimics bankart lesions: a magnetic resonance imaging-based study. Arthroscopy. 2020 Feb;36(2):336-344.
- Kelly A, Heath MR, Amoroso EE, et al. Normal humeral head ossification in pediatric and adolescent shoulders can Mimic Hill-Sachs lesions: a magnetic resonance imaging-based study. J Pediatr Orthop. 2022 Feb 1;42(2):e143-e148.
- Hurley ET, Davey MS, Montgomery C, et al. Arthroscopic bankart repair versus open latarjet for recurrent shoulder instability in athletes. Orthop J Sports Med. 2021 Sep;9(9):23259671211023801.
- Little DG, Sussman MD. The Risser sign: a critical analysis. J Pediatr Orthop. 1994 Sep-Oct;14(5):569-575.
- Charles YP, Dimeglio A, Canavese F, et al. Skeletal age assessment from the olecranon for idiopathic scoliosis at Risser grade 0. J Bone Joint Surg Am. 2007 Dec;89(12):2737-2744.
- Troy MJ, Miller PE, Price N, et al. The “Risser+” grade: a new grading system to classify skeletal maturity in idiopathic scoliosis. Eur Spine J. 2019 Mar;28(3):559-566.
- Risser JC. The Iliac apophysis; an invaluable sign in the management of scoliosis. Clin Orthop. 1958;11:111-119.
- Pennock AT, Bomar JD, Manning JD. The creation and validation of a knee bone age atlas utilizing MRI. J Bone Joint Surg Am. 2018 Feb 21;100(4):e20.
- Meza BC, LaValva SM, Aoyama JT, et al. A Novel shorthand approach to knee bone age using MRI: a validation and reliability study. Orthop J Sports Med. 2021 Aug;9(8):23259671211021582.
- Nicholson AD, Liu RW, Sanders JO, et al. Relationship of calcaneal and iliac apophyseal ossification to peak height velocity timing in children. J Bone Joint Surg Am. 2015 Jan 21;97(2):147-154.
- Garcia MR, Nicholson AD, Nduaguba AM, et al. Ossification of the phalanges of the foot and its relationship to peak height velocity and the calcaneal system. J Child Orthop. 2018 Feb 1;12(1):84-90.
- Nelson S, Hans MG, Broadbent BH, Jr., et al. The brush inquiry: an opportunity to investigate health outcomes in a well-characterized cohort. Am J Hum Biol. 2000 Jan;12(1):1-9.
- Li SQ, Nicholson AD, Cooperman DR, et al. Applicability of the calcaneal apophysis ossification staging system to the modern pediatric population. J Pediatr Orthop. 2019 Jan;39(1):46-50.